
Omniya is an award winning luxury clinic, with a core focus on internal and external health and well-being. We are London’s first clinic, specialising in helping you feel better, inside and out. We are proud to host some of the UK and Europe’s leading doctors and practitioners for:
External Health: Botox, dermal fillers, facials & hydrafacial, BBL Moxi, laser hair removal and other aesthetic and skin treatments, performed by London’s leading surgeons, doctors and aestheticians, and
Internal Health: hormone replacement treatment (HRT) for the treatment of menopause, and testosterone replacement treatment (TRT) for the treatment of andropause.

At Omniya Clinic, we offer personalised treatments, tailored to your needs by combining our advanced techniques, technologies and commitment to professionalism.

At Omniya Clinic we work with some of the leading practitioners in their area of work, who are highly qualified with recognised credentials and specialised training.

We offer free face and body consultations to better understand your needs and goals allowing us to recommend and tailor treatments for you.
*available with participating practitioners
To find out more about our range of treatments and prices, visit our pricing page below.
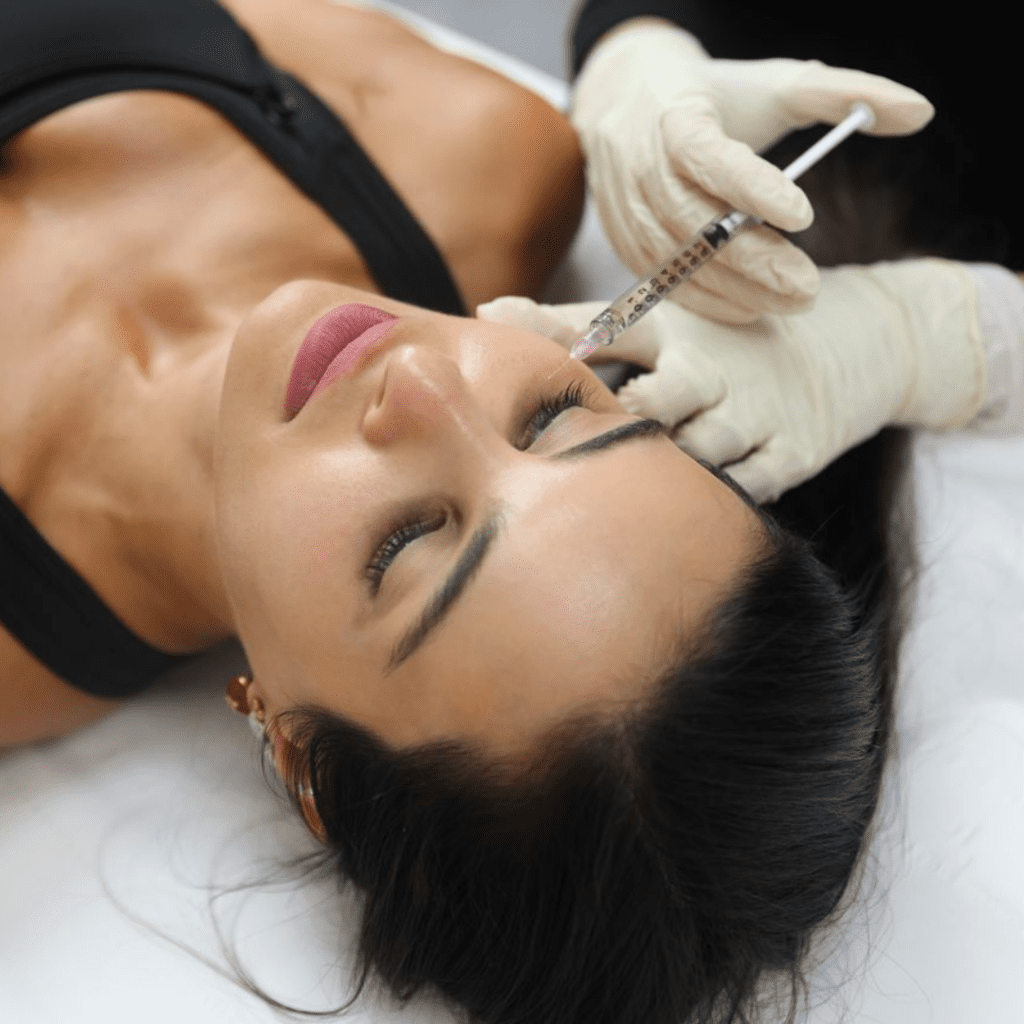

Known as anti-wrinkle injections, Botox can be administered in several areas on your face and body, primarily used to restore a youthful appearance, effectively improving signs of ageing.
Read moreDermal fillers are versatile anti-ageing solutions, effective in treating deep lines, wrinkles, and volume loss, catering to both men and women by providing tailored treatments to contour and lift facial features.
Read moreWe take pride in delivering the best facials in London, incorporating machine technology and expert hands-on techniques to ensure long-lasting results performed by our highly experienced aestheticians.
Read moreHormone therapy offers a wide range of significant health benefits by inducing physiological changes in the body, and enhancing cardiovascular, neurological, and bone health, while also improving symptoms and overall quality of life.
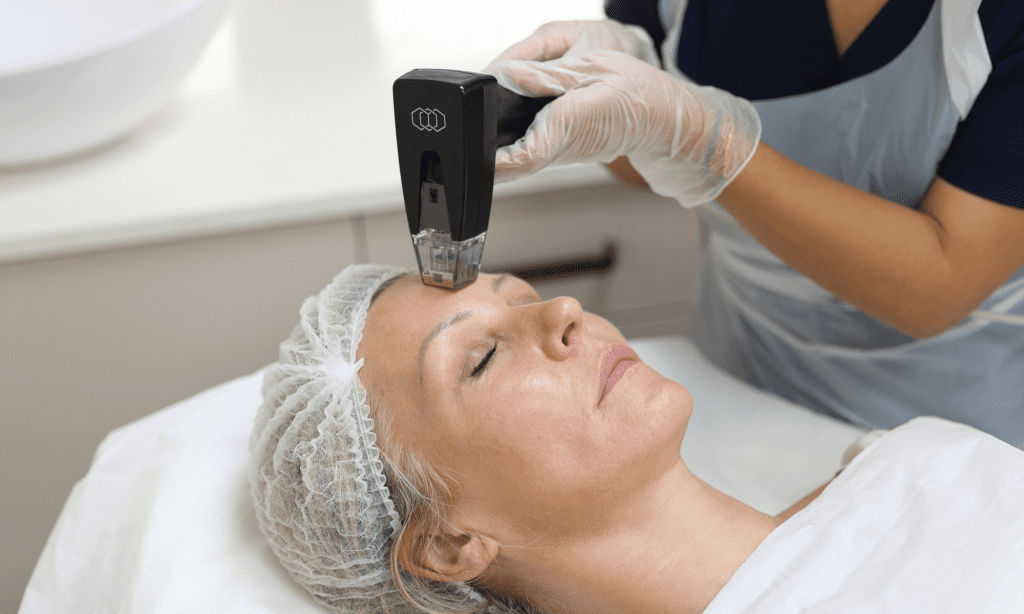
Read moreMorpheus8 is a radiofrequency microneedling device that enhances body and facial contours, by addressing wrinkles, acne scars, and discolouration. Omniya proudly provides Morpheus8 treatments at their London Clinic in Knightsbridge.
Read moreLaser hair removal eliminates the hassle of frequent leg waxing and the stubble from razor blades, giving you a permanent solution for smoother, clearer skin and reducing ingrown hairs for a fantastic overall appearance.
Read moreOur team of experts are here to help you by ensuring we offer treatments that will help with your concerns, insecurities or achieve your aesthetic goals.

All treatments are performed by qualified experts in our London Clinic.

At Omniya Clinic, all treatments are performed in our treatments rooms using only the very best equipment on the market.

During your consultation, your specialist will work with you to understand your needs before recommending treatments and expert advice.













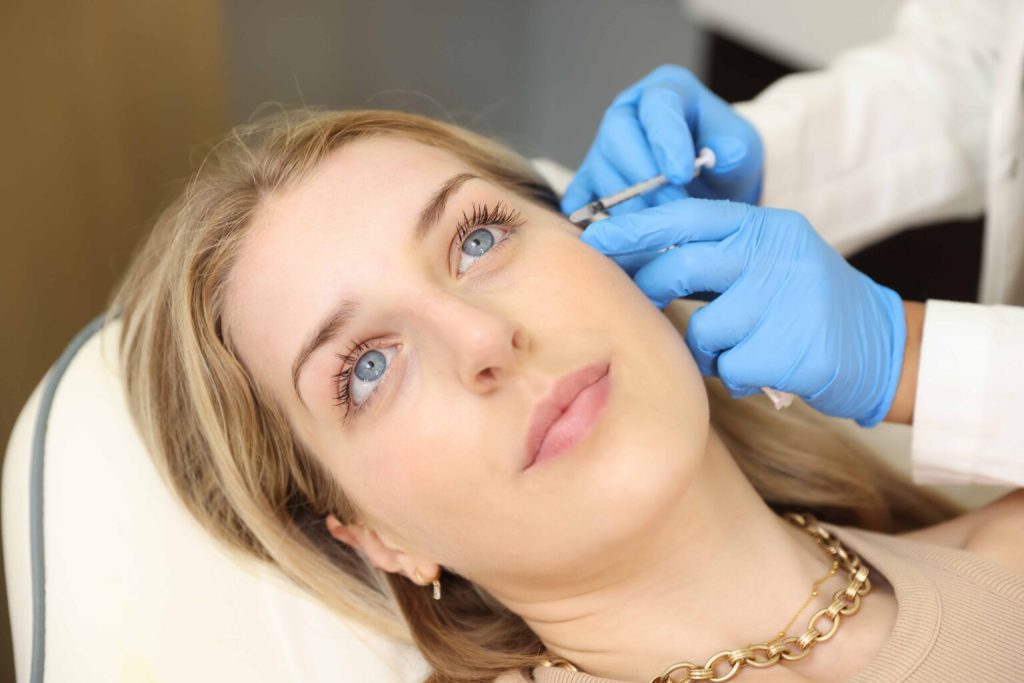
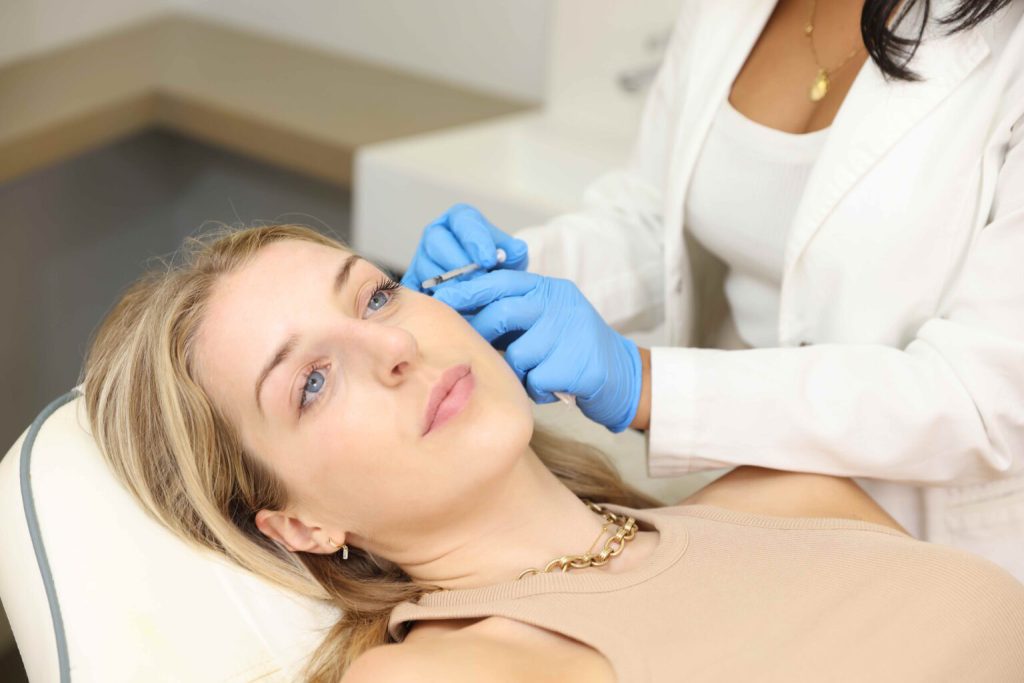



If you are an existing patient, we kindly request that you email your request through to hello@omniya.co.uk rather than filling out the contact form.
"*" indicates required fields